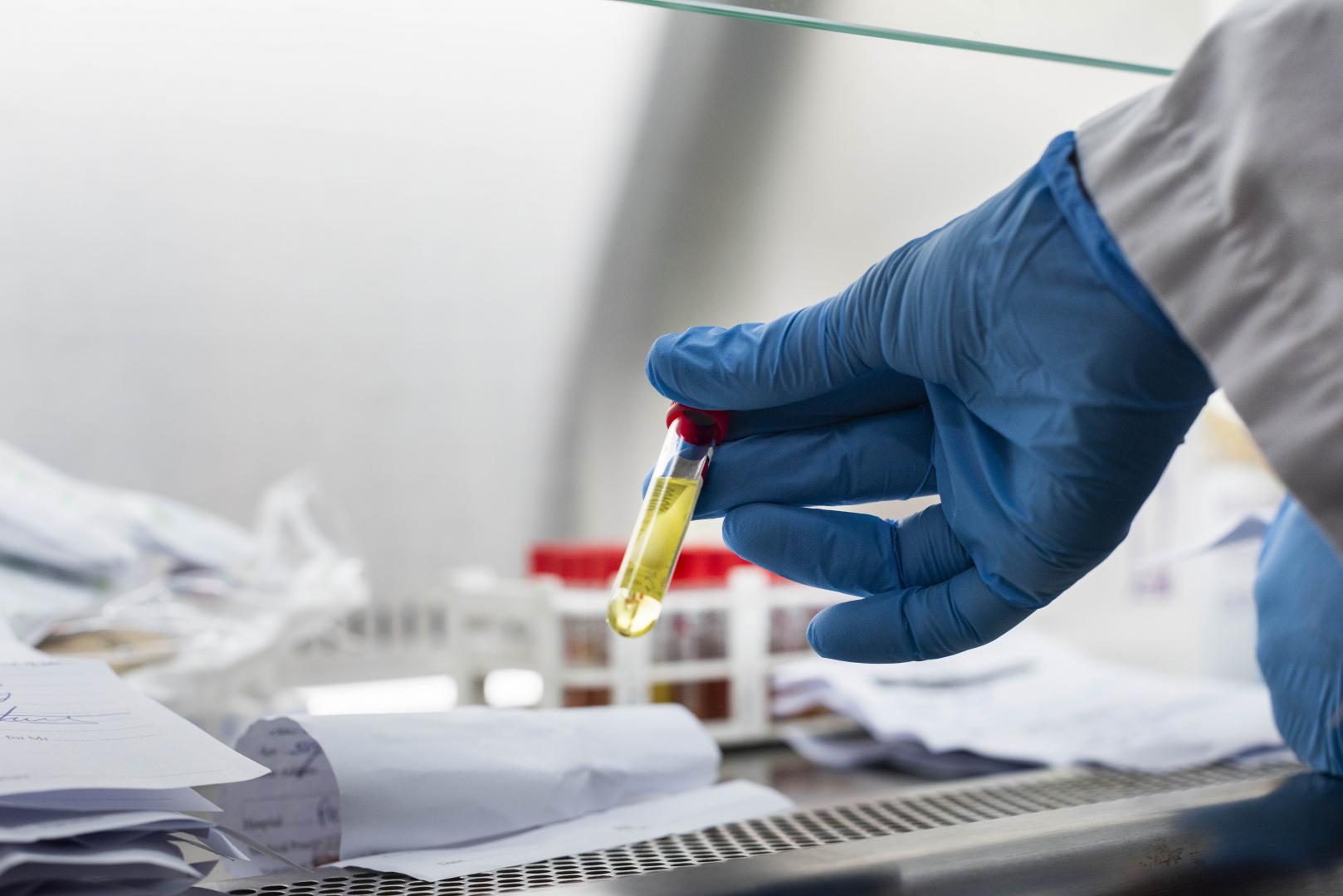
Trials for a leading Covid-19 vaccine candidate, ChAdOx1 nCoV-19, officially began in Kenya in Kilifi County on Friday, October 30.
The vaccine was developed by the University of Oxford in collaboration with AstraZeneca. Trials are ongoing in countries including Brazil, South Africa and the United Kingdom.
In Kenya, the trials are being undertaken at the KEMRI-Wellcome Trust Research Programme base in Kilifi. The long-running research partnership brings together the Kenya Medical Research Institute (KEMRI), Welcomme Trust and the University of Oxford.
Already, a first batch of volunteers has been vaccinated after requisite approvals were secured from the Ministry of Health and the Kilifi County Government.
The trial is targeting 400 volunteers for vaccination, with 40 front-line workers based in Kilifi County among those already identified.

There is a likelihood of the trial being expanded to neighbouring Mombasa County.
Vaccinated volunteers will be assessed over a twelve-month period, with researchers looking out for any side-effects.
They will also be observing how bodies build immunity against Covid-19 once the vaccine is administered.
READ>>>>>KU Students Win UN Person of the Year Award for Covid-19 Innovation
With trials being conducted for the vaccine in different parts of the world, it remains crucial to observe how it affects Kenyans in particular.
“Vaccines which work in one population do not necessarily work in all populations; this has been witnessed in the case of vaccines against malaria, rotavirus and Ebola. To ensure that Kenyans can benefit from the ChAdOx1 nCoV-19 vaccine if it proves to be successful, it is important to assess its performance among Kenyan volunteers,” the researchers explained in a statement.
Countries around the world including Russia, the United States and the United Kingdom have been racing against time to develop a vaccine for the virus.
A safe vaccine with approval from relevant authorities is considered vital in a world turned upside down by the effects of the pandemic.
In August, Russia became the first country in the world to approve a Covid-19 vaccine, dubbed Sputnik.
A second vaccine was unveiled by Russia in October, dubbed EpiVakKorona.
While reports from Moscow indicate that the country could begin mass vaccination as early as the end of the year, doubts linger on whether the vaccine has undergone the rigorous thee-phase trials required in other jurisdictions.


















































![Pula Co-Founders and Co-CEOs, Rose Goslinga & Thomas Njeru. Pula provides agricultural insurance and digital products to help smallholder farmers manage climate risks, improve farming practices and increase their incomes. [ Photo / Courtesy ]](https://businesstoday.co.ke/wp-content/uploads/2021/01/Pula-Co-Founders-and-Co-CEOs-Thomas-Njeru-Rose-Goslinga.jpg)























































Leave a comment