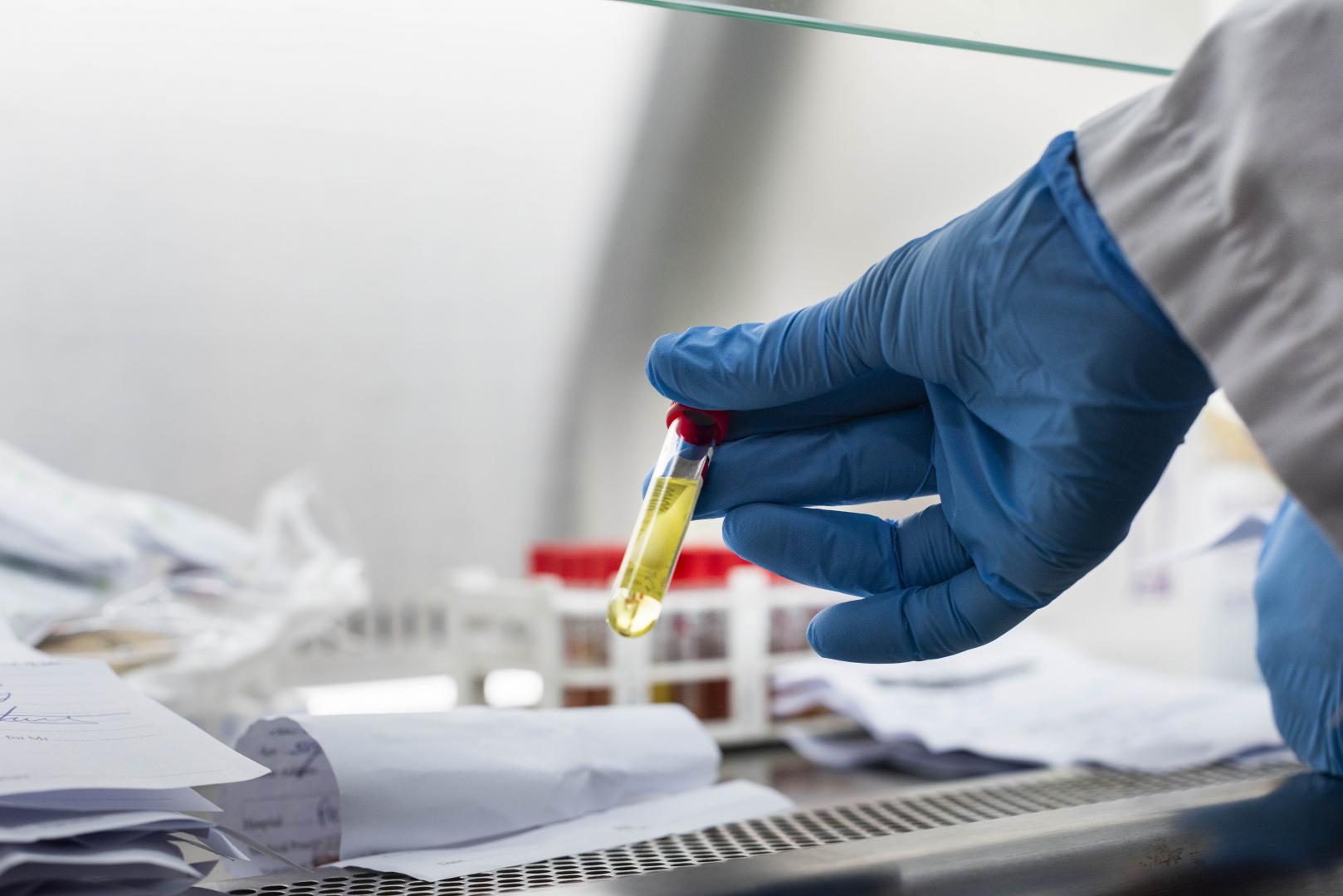
The World Health Organization (WHO) estimates that about 10 per cent of global pharmaceutical supply is counterfeit and substandard. Yet the percentage of substandard medicines in Sub-Saharan Africa is even higher, if we are to go by the figures indicated in the Journal of Pharmaceutical Policy Practise published in November 2016.
According to an article published in the journal, “Fighting poor-quality medicines in low and middle income countries: The importance of advocacy and pedagogy”, 34 per cent of medicines in Sub-Saharan Africa are of poor quality.
Indeed, there is increasing globalisation of pharmaceutical production. Pharmaceutical ingredients and finished products are produced in many different regions and countries before being moved to the international market through multiple distribution channels. However, as this continues, the global shift has not been accompanied by a strengthening and harmonization of the regulatory systems. Consequently, the global pharmaceutical market is typified by numerous standards of quality.
In April 2015, leading health researchers and regulators were unanimous that poor quality and falsified medicines are potentially devastating to global health. Writing in the American Journal of Tropical Medicines and Hygiene, the experts agree that globalization has redefined the field of medical product regulation. Quality of medicine is one of the main concerns of health care providers and patients. It also happens to be a huge public health challenge in developing countries.
The risks posed by using poor quality medicines are enormous. Any medicine, if not used properly is dangerous.
When patients are given medicines that are not effective in treating their conditions, they are likely to develop resistance towards micro-organisms that these medicines are supposed to treat, not to mention that their condition is likely to worsen. Others may react adversely to the medicines. Normally, adverse medicine reactions occur when patients are given medicines whose contents or active ingredients do not target the intended disease but instead cause unforeseen reactions.
Looking at the national welfare and economies, it is undeniable that a healthy nation is a wealthy nation. If the working population of a country is weakened by usage of counterfeit drugs, then there will be corresponding weakening towards nation building and wealth creation. Also, a market flooded with counterfeit and substandard medicines kills the pharmaceutical industry, as this denies local manufacturers opportunity for fair market competition and growth in the overall economy. Suffice is to say that substandard or counterfeit medicines also kill.
A 2015 study jointly conducted by the Ministry of Health and the World Bank indicated that patients were being treated with bad medicine in some government hospitals and clinics.
[crp]
The study revealed that patients in Nairobi are routinely given drugs that include antibiotics and painkillers that do not meet the required standards. The study was based on 300 prescriptions collected from 42 health facilities and retail pharmacies across Nairobi. Seventeen per cent of these samples failed quality tests at the Kenya National Quality Laboratory.
Government and other players in this industry need to ensure that patients have access to good quality medicine. Before admitting suppliers, organisations should take them through very stringent pre-qualification processes. As part of the Quality Assurance Protocol, MEDS, for instance, tests pharmaceutical products received from its supply chain to ascertain quality at its World Health Organization (WHO) Prequalified Laboratory.
In collaboration with Pharmacy and Poisons Board (PPB) and the Kenya National Quality Laboratory, drug suppliers can play a key role in the fight against counterfeit and substandard medicines.
Since a WHO prequalified laboratory operates at international standards and is used by United Nations (UN) affiliated agencies among others to test the quality of medicines, its results can be used to make important decisions that would help in the fight against substandard and counterfeit medicines.
Dr Jane Masiga is the Head of Operations at Mission for Essential Drugs and Supplies. Email: [email protected]


















































![Pula Co-Founders and Co-CEOs, Rose Goslinga & Thomas Njeru. Pula provides agricultural insurance and digital products to help smallholder farmers manage climate risks, improve farming practices and increase their incomes. [ Photo / Courtesy ]](https://businesstoday.co.ke/wp-content/uploads/2021/01/Pula-Co-Founders-and-Co-CEOs-Thomas-Njeru-Rose-Goslinga.jpg)
























































Leave a comment