Combustible cigarettes have been named by the Center for Disease Control and Prevention among the number one causes of cardiovascular/respiratory diseases and also the basis for several other health risks for both active and passive smokers for over 30 years. But new innovations like the recently FDA approved IQOS Tobacco Heating System with ‘Reduced Exposure’ and the Oral Tobacco Heated Snus have disrupted the tobacco ecosystem.
These innovations have presented hope for smokers and led to a global exchange of views, ideas and reactions among public health experts, government representatives, investors, members of the tobacco/nicotine industries, global media round tables and several other tobacco smoking-related forums.
In the 1950’s, smoking hazards were covered up by the tobacco industry public relations machinery, according to research findings by Tobacco explained. In recent years, there’s has been a wave of instances where eminent tobacco producing companies like British American Tobacco and Philip Morris International have come out in various ways to join with stakeholders and face the effects that nicotine causes and to also work towards elucidations with a belief that deepening the conversation about tobacco, nicotine and public health can lead to more informed views and decisions by all beneficiaries.
There is need for public and private collaborative strategies to substantially contribute to less harm in society, as highlighted during the recent Wall Street Journal webinar. Dr Moira Gilchrist, PMI Vice President, Strategic and Scientific Communications championed for key stakeholders in the tobacco industry to discuss the trending modified risk tobacco products and communicate their relative risks and debate on possible outcomes for the good of society.
She calls for open dialogue on whether the new innovations will eventually be the long-awaited solution for the “tobacco harm reduction” concept that includes lowering nicotine addiction, preventing usage in youth, lowering health problems and mortality rate that occurs as a result of smoking combustible cigarettes and overtime total withdrawal from usage: and whether or not the new products will actually serve the claimed purpose and lead to many other innovations to achieve a smoke-free world.
Below is a Q&A with Dr Moira from another event, the Global Tobacco Nicotine Forum, where she provides details on issues regarding the current innovations, the future of combustible and non-combustible cigarettes, and tobacco regulations around the world.
QUESTION: What is the significance of the U.S. Food and Drug Administration (FDA) approval for PMI to market IQOS, as a Modified Risk Tobacco Product (MRTP)?
ANSWER: It really marked an important and somewhat historic milestone. For the first time, a novel product received MRTP orders with reduced exposure claims in the U.S and I think it’s a really important precedent for demonstrating to everybody that not all tobacco products are the same in 2020. This is something we’ve known scientifically for many years now as we were building the evidence package for IQOS and I am really grateful now that the FDA went through the rigorous process of reviewing all our science and came to the decision to grant the orders that they did. Now is the time for other countries to have a look at what FDA has done and think what it means for their own tobacco control policy and think what it means to not only bring better products but also information that would help guide their choices.
When do you think other countries will follow suit following the FDA’s example?
In my view and in my company’s view, the time is now. These innovations exist, and we know that science demonstrates that there are better alternatives than continuing to smoke for those who switch completely so there’s no reason for delay. There’s no reason to be thinking about this any longer, we know from the example that the FDA has set that it’s possible to bring these products onto the market, to allow communications to adults who smoke, and to also put in place these guard rails that ensure once the product is on the market, there aren’t worrisome levels of unintended consequences like for example youth use.
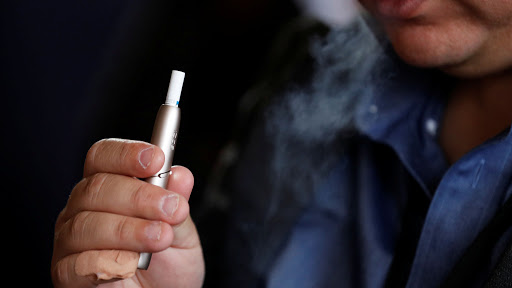
That’s exactly what the FDA has done, it didn’t stop at the decision, they put in place all processes and guidelines that we have to follow in order to keep the product on the market and I think other countries can certainly learn from that approach.
How do you think smokers around the world should respond to the FDA announcement on MRTP?
Smokers are all generally interested in alternatives to continue to smoke. I think there a high level of interest in smokers all around the world and what smokers can do is to inform themselves about the products, the better choices that exist today. To be clear these products are not a replacement for quitting, the best thing any person who smokes can do is to quit altogether but we also know that the vast majority of smokers in any country in any given year do not quit so they should arm themselves with information about the better choices that exist today, and ones that are backed up by science like IQOS.
Do you expect public health officials and regulators to behave differently and regulate differently from now on?
I would just encourage those involved in the regulation or the policymaking for innovative new alternatives to cigarettes to really think about the policies that are in place in their country and to really think about whether those policies clearly differentiate between the most harmful form of nicotine consumption, which is through cigarettes on one hand which should unquestionably be subject to strict regulation, and on the other hand better alternatives that are shown by science to even reduce people’s exposure to harmful chemicals compared with continuing to smoke or to reduce their risk in other ways.
They should think carefully about how their regulation distinguishes between those two because how can a person who smokes make a better choice if they don’t know the product exists, or if they don’t know that it’s different from continuing to smoke. It is only through the provision of truthful information and access to products that I believe we can really have a measurable impact on public health in a very short space of time.”
Do you think harm reduction with alternative products such as electronic products that are expensive can work in lower-income countries?
Of course, harm reduction is for each and every adult who smokes anywhere in the world and that’s why we need a range of different products to suit different adult’s preferences. Some people prefer electronic cigarettes, some people prefer heated tobacco products, and others prefer oral tobacco products like Snus. Within those ranges of different products, there are products with different price categories and I think that’s really important and as a company, we are focused on bringing all these products not just to the developed countries but to middle-income countries to make sure everybody has access to better alternatives.”
Can we trust the science of PMI?
I get this question often and my answer is always the same, you don’t need to trust because we’ve made it all public! You can scrutinize the data, you can re-analyze the raw data if you like you can look at all of our applications to the USFG. On FGA’s website there are hundreds of thousands of pages of science, there’s science all over our PMI science website, we’ve published in peer-review journals, you don’t need to trust you can just go and scrutinize yourself and I think that’s a really important fundamental principle, to be really open and transparent so people can come to their own conclusions.”
What is the difference between your non – combustible products and the ones of other companies and have they got the same science behind them?
“I can’t speak for other companies but I can speak for my company, I think we’re the company that has probably invested the most in not only the development of products but also in cutting-edge science. That is why we feel that it is important to substantiate the potential of these products and we’ve been doing this for more than a decade and not just on heated tobacco products.
New tobacco technology innovations will cut risks as the current science and research is indicating,
PMI also has an e-vapor e-cigarette product in our portfolio. I think the proof point about investment and the proof point of the seriousness and the quality of our approach is really the FDA decision from July this year so we’ve been able to meet an extremely high bar that was set by the tobacco control act in the United States, our science withstood scrutiny and the IQOS product Is the first and only electronic product to have been granted modified risk tobacco product status with reduced exposure order that the FDA granted.”
What can consumers do to advocate for access to better choices?
Many consumers are very well organised and vocal. We saw, for example, in the United States a campaign last year to try and protect the rights of adult smokers to access vaping products. I would just encourage people who smoke all around the world to look and see examples in other countries and see how smokers and tobacco products users are organizing themselves to fight for their fundamental right to access the products which are better choices than continuing to smoke.
With all the current shifts in tobacco consumer behavior, progress requires attention to market forces. New tobacco technology innovations will cut risks as the current science and research is indicating, but also smarter regulators like the FDA are supporting smokers in switching to tobacco harm reduction products, as a result, science-based information on the true benefits and risks will create demand to change leaving companies to compete in lowering risks via alternative nicotine products. Rapid progress toward tobacco harm reduction and smoking cessation is to be expected over time.











Leave a comment